Geochronology – Radioactive Dating Methods
June 6, 2014
Geochronology
Radioactive Dating Methods
Introduction
This is a brief summary of the presentation given to Reasonable Faith Adelaide on Thursday 15th of May by Dr Justin Payne. See our You Tube recording for the complete talk and discussion. See also his Geochronology Power Point Slides.
Justin was a lecturer and researcher within the School of Earth and Environmental Sciences at the University of Adelaide, but is now working for the University of South Australia. His presentation was part of our series on Old earth versus Young Earth Creationism (YEC). Justin presented the conventional scientific view on dating methods, which supports an old earth.
Geochronology is the science of determining the age of rocks, fossils, and sediments, within a degree of uncertainty inherent to the method used. Geochronology is a generic term for dating methods. However, in practice it is closely associated with radioactive dating methods. Justin’s talk concentrated specifically on Uranium/Lead dating using Zircon crystals.
Basics of Radioactive Decay
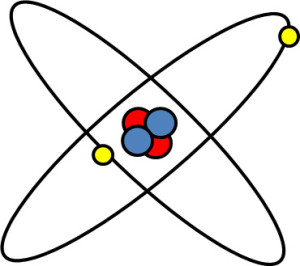
The nucleus of each atom contains protons and neutrons. Both protons and neutrons are called hadrons. Protons are positively charged and neutrons do not have any charge. An atom contains an outer shell of electrons. If an atom is neutrally charged then the number of electrons equals the number of protons. It is the number of electrons that determines the chemical behaviour of each element. An image of a Helium atom is shown below.
The nucleus of a Helium atom contains 2 protons (red) and 2 neutrons (blue). The nucleus is surrounded by 2 electrons. The atomic number of an element is the number of protons in the nucleus and the atomic weight is the number of hadrons (protons plus neutrons). Thus for a Helium atom the atomic number is 2 and the atomic weight is 4.
Each Uranium atom has 92 protons and so its atomic number is 92. Uranium has several isotopes. The common isotopes are 235U and 238U, where U is the symbol for Uranium and the superscripts indicate the number of hadrons (atomic weight). Uranium isotopes are unstable and undergo radioactive decay. When the elements decay they emit particles or rays and form different elements. When a nucleus decays it emits an:
- An Alpha particle,
- Beta particle, and/or
- Gamma ray.
An alpha particle is a Helium nucleus. Thus when a nucleus emits an alpha particle the nucleus loses 2 protons and 2 neutrons. This means that the atomic number is reduced by 2 and the atomic weight is reduced by 4. A Beta particle is simply a negatively charged electron. When a nucleus emits a beta particle, this means that a neutron is changed into a proton. So the atomic number is increased by 1 and the atomic weight remains the same. A gamma ray is a high energy electromagnetic wave (photon) and does not change the atomic weight or atomic number.
Uranium/Lead decay
Justin focussed on Uranium/Lead decay within Zircon crystals.
Zircon crystals are small crystals that form within rocks, such as granite. In its molten state, Zircon rejects lead. Thus, when Zircon crystals are formed, they will not contain any lead. Thus the age of a Zircon crystal can be dated from the Uranium/Lead ratio.
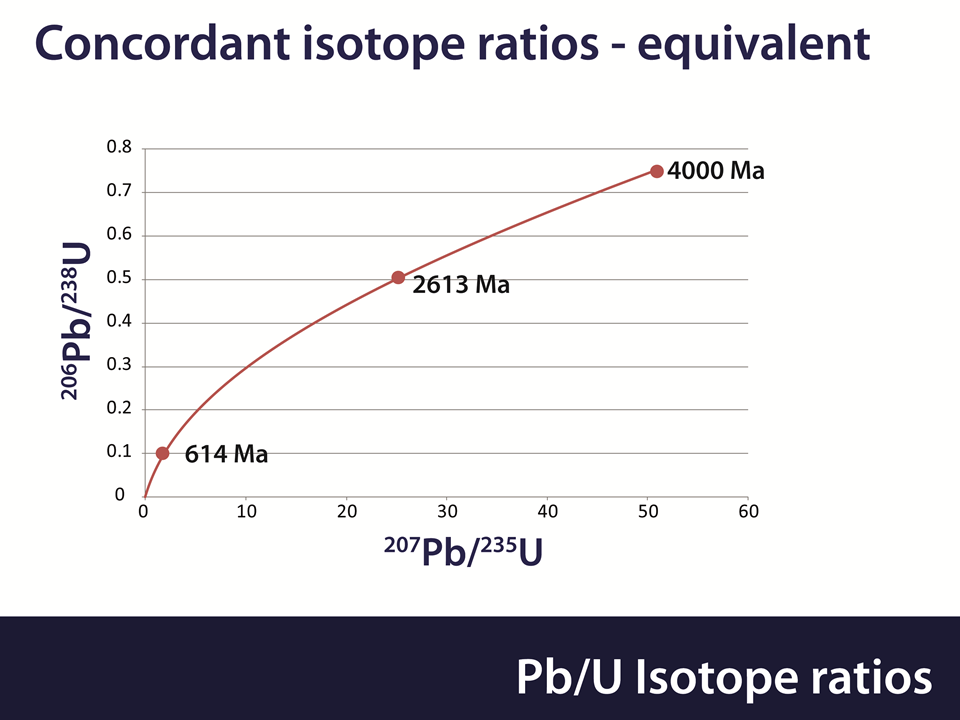
Uranium exists in 2 isotopes and these have different half lives. 238U decays into 206Pb with a half life of 4.47 billion years and 235U decays into 207Pb with a half life of 0.704 billion years. The age of a Zircon crystal can be calculated from the Lead/Uranium ratio for both isotopes. Both of these calculations are independent and should yield the same answer. This is expressed by the Concordia diagram as shown below.
If all is well then the Lead/Uranium ratios should match each other on the red line. If they do not, then an “event” has occurred in the past, such as loss or gain of either Lead or Uranium. Methods exist for determining how long ago this event occurred.
The age of the crystal can also be estimated from the ratio of the Lead isotopes. If there is a Lead loss then this will apply equally to both Lead isotopes. Thus age estimates from the Lead isotope ratios are unaffected by Lead loss.
Some Zircon crystals have defects that yield false results. However, these defects are detectable and so scientists have a reasonable idea for knowing when to reject test samples.
Justin has been dating Zircon crystals for a considerable time in diverse areas in Western Australia and South Australia. He also does the analysis of the zircon samples in laboratory facilities at the University of Adelaide. Over time the results yielded are generally consistent and affirm confidence in the technique. The claimed accuracy of the technique is better than 1%.
Young Earth Creationists claim that radioactive dating makes the following assumptions:
- The decay rate has been constant throughout time.
- The isotope abundances in the specimen have not been altered during its history by the addition or removal of either parent or daughter elements.
- When the rock was formed it contained a known amount of the daughter material.
The first item is indeed an assumption. However, there is no evidence to the contrary and the decay rates conform with atomic theory.
The second item is not an assumption. Methods do exist for detecting loss or removal of the parent or daughter elements and also for estimating when these events occurred.
The third item is also not an assumption. For Uranium/lead dating, molten Zircon rejects Lead. This can be tested in the laboratory in the here and now. Hence, the initial composition of the Zircon crystal is known.
Justin claims that Young Earth Creationists (YECs) highlight the minor instance where dating methods do not work and do not properly acknowledge the majority of cases where they do. Geochronologists are aware of the assumptions in the method and of possible causes of erroneous readings.
This is a very simplified summary of Justin’s presentation. I suggest that you watch the You Tube recording of his presentation and the subsequent discussion.
Kevin Rogers